Since the inception of In Vitro Fertilization (IVF), several million babies have been born worldwide as a result of this treatment. Assisted Reproductive Technologies (ART) are a group of treatment options used for couples with infertility that cannot be treated by simpler methods.
Understanding the applications of each procedure will help you get the right treatment and maximize your chances of success. There are several different types of treatments included under the umbrella of ART. The main treatment is IVF, and it is important to understand the four basic components of an IVF cycle: Ovarian Stimulation, Egg Retrieval and Fertilization, Embryo Culture, and Embryo Transfer.
WHO ARE CANDIDATES FOR IVF?
For some situations or conditions, such as tubal factor, IVF may be the first-line treatment. In other cases, IVF is recommended only if simpler treatment fails. Below is a list of common indications for IVF treatment.
A-TUBAL DAMAGE
The only options to treat significant damage to the tubes are surgical repair or omission of the tubes with IVF. This decision should be carefully individualized in each situation.
B-ENDOMETRIOSIS
Endometriosis can be effectively treated with a combination of surgical and medical therapy. IVF is effective as a second-line treatment if initial treatment fails. Learn more about endometriosis and its treatments in our article on understanding endometriosis in adolescents and the usefulness of performing surgeries before IVF.
C-ANOVULATION AND PCOS PATIENTS
The only options to treat significant damage to the tubes are surgical repair or omission of the tubes with IVF. This decision should be carefully individualized in each situation.
D-MALE FACTOR INFERTILITY
One of the most dramatic advances in infertility treatment has been the ability to achieve fertilization and pregnancy in the IVF laboratory with severely abnormal sperm samples using Intracytoplasmic Sperm Injection (ICSI). ICSI is often recommended if there is any indication of a sperm problem, if sperm is obtained surgically, or if there has been a previous fertilization failure.
F-AGE-RELATED INFERTILITY
Age is considered the primary predictor of success in fertility therapy. In normal reproductive life, a woman’s ovarian function declines with age. In many cases, this reduced function can be overcome by the use of IVF alone or in conjunction with techniques such as PGT and its variants and blastocyst culture.
G-UNEXPLAINED INFERTILITY
Approximately 20% of couples will not have an identifiable cause of infertility after completing a comprehensive evaluation. IVF often succeeds even if more conservative treatments have failed, especially because some of these couples actually have some blockage to fertilization.
STEPS OF AN IVF CYCLE
MICROMANIPULATION TECHNIQUES
1. OVARIAN STIMULATION FOR EGG DEVELOPMENT
Controlled “superovulation” techniques used in IVF are designed to stimulate the ovaries and produce several eggs instead of the single egg normally produced in a natural cycle. Multiple eggs increase the potential availability of several embryos (fertilized eggs) for transfer and ultimately increase the likelihood of conception.
The medications needed to stimulate egg production may include, among others: Lucrin (gonadotropin-releasing hormone – agonist), Cetrotide (gonadotropin-releasing hormone – antagonist), Gonal-F (follicle-stimulating hormone), Merapur and Pergoveris (combination of FSH and LH, luteinizing hormone, or recombinant FSH and LH), and Ovidrel (hCG, recombinant human chorionic gonadotropin). Each is administered by injection only. Most medications are administered subcutaneously (under the skin), although some are intramuscular injections (into the muscle).
The risks associated with injectable fertility medications may include, but are not limited to, sensitivity, infection, hematoma, and swelling or bruising at the injection site.
2. EGG RETRIEVAL (EGG RECOVERY)
For IVF, egg retrieval is usually done under transvaginal ultrasound guidance. To achieve this, a needle (under intravenous sedation) is inserted through the vaginal wall into the ovaries using ultrasound to locate each follicle.
Follicular fluid is aspirated into a test tube to obtain the eggs. Although patients are given pain medications intravenously and are carefully monitored by an anesthesiology team, some women may experience some discomfort during the procedure.
Overall, egg retrieval takes 20 to 30 minutes. Usually, patients are discharged home a few hours after retrieval.
3. SPERM COLLECTION AND PREPARATION
A semen sample will be obtained from the partner through masturbation on the day of egg retrieval. This is usually obtained while the retrieval is being performed. Abstinence from ejaculation for two to five days before providing this semen sample is recommended. After the sample is obtained, the sperm will be prepared for inseminating the collected eggs in our laboratory.
Since this can be a stressful time for men, the man/couple may not be able to produce a sample when needed.
Men who feel they may have difficulty producing a semen sample have the opportunity to freeze their samples in our laboratory in advance for use in this situation. Testicular biopsy can also be performed as a method for extracting sperm for IVF.
4. EGG INSEMINATION AND EMBRYO CULTURE
After egg retrieval, the follicular fluid is immediately moved to the adjacent laboratory to identify, evaluate, and prepare the eggs for insemination.
Fertilized eggs will be allowed to develop for 3, 4, or 5 days or more additional days under controlled laboratory conditions before being placed into the woman’s uterus. Depending on the couple’s wishes, some fertilized eggs/embryos may be frozen, or biopsied for the search for chromosomal or genetic diseases of the embryo, and stored for future use. They will also be vitrified in case of the production of multiple eggs or some other specific circumstances.
After the embryos are transferred to the uterus, the woman will continue with progesterone supplementation that begins in the afternoon of the egg retrieval procedure. Progesterone can be administered in the form of vaginal suppositories or by injections. The administration of these medications after egg retrieval has been shown to create a more favorable uterine environment for the embryos, increasing pregnancy rates.
5. EMBRYO TRANSFER TO THE UTERUS
Embryos are transferred on the third or fifth day of development. FERTILITE embryologists are highly skilled in identifying “healthy” embryos and, in 95% of cases, will recommend that a patient extend embryo development to day five, known as the blastocyst stage, as it can increase the chances of success by reducing the likelihood of multiple pregnancies.
Embryos are transferred to the uterus through a small tube (catheter). This procedure is very similar to a vaginal cytology and does not require anesthesia and is generally painless. The embryos are placed in a small amount of fluid inside the catheter, which is passed through the cervix at the time of a speculum examination. The embryos are placed so that they reach the upper part of the uterus. The number of embryos transferred depends on the individual circumstances of the couple, and this decision will be made collectively by you, your doctors, and the embryologist. Normally, a maximum of embryos is transferred in one treatment cycle.
After the transfer, the woman can dress and leave after a brief recovery period. A pregnancy test will be done twelve to fourteen days after the transfer, regardless of whether uterine bleeding occurs or not.
Transferring several embryos increases the likelihood of success. Transferring multiple embryos also increases the risk of a multiple pregnancy. Any multiple pregnancy carries a higher risk of spontaneous abortion, premature delivery, and premature birth, as well as a higher financial and emotional cost.
ALTERNATIVES IVF-ET:
Depending on the cause or causes of infertility for each individual and unique couple, there may or may not be the possibility of conception through other means, such as intrauterine insemination (IUI), in addition to IVF-ET. The possible success rates of these alternatives may vary depending on the type and severity of the cause of infertility. For some couples, it may even be possible to conceive spontaneously without the help of a physician. You should discuss these alternative treatment methods with your doctor before proceeding with IVF-ET therapy.
MICROMANIPULATION
INTRODUCTION
Since the historic milestone in 1978 when the first birth through “in vitro” conception outside the human body was achieved, the fundamentals of fertilization and early development have maintained their essence: the creation and maintenance of a sterile and highly controlled environment to allow fertilization and embryonic development without hitches. This methodology has evolved over two decades, providing a greater understanding of its nuances and the opportunity to apply it in an increasing variety of infertility cases. Notable advances have led to the design of more effective culture media, allowing for the prolonged “in vitro” embryonic development. Furthermore, surplus embryos, and in some cases, eggs, can be cryopreserved using liquid nitrogen for future pregnancy attempts. However, fertilization is no longer a random occasion thanks to the introduction of assisted micromanipulation. This technique allows for precise manipulation of embryos, facilitating embryo biopsy for evaluating its genetic profile and enhancing its implantation potential by assisting in hatching through microperforations in the embryo’s outer layer.
CONVENTIONAL IN VITRO FERTILIZATION (IVF)
Conventional In Vitro Fertilization (IVF) involves controlled ovarian hyperstimulation to recover mature eggs from a woman’s ovary. The number of eggs recovered can vary, from one to more than thirty, depending on how the ovaries respond to the gonadotropins used for their stimulation.
Mature eggs are collected in a balanced saline solution and maintained at 37 °C until insemination. At the same time, a semen sample is processed to separate and obtain clean and active sperm. In the IVF laboratory, ensuring the correct combination of sperm and eggs is emphasized through labeling and verification. After a few hours of culture, eggs and sperm are mixed to allow fertilization. However, the fertilization rate can vary depending on the quality and maturity of the eggs and sperm, meaning that the initial number of eggs does not guarantee the same number of embryos.
The in vitro culture period of embryos is crucial for determining how many embryos will be used in the IVF cycle. This can last from one day to seven days in the case of blastocysts. If conditions are optimal, embryos are typically cultured during their early stages to select the most viable ones for transfer or cryopreservation. Blastocysts are the last stage before implantation in the uterus. Culturing embryos to the blastocyst stage improves selection and reduces the need to transfer multiple embryos. With fewer transfers of three or more embryos, the risk of multiple pregnancies decreases, which in turn reduces the risks of pregnancy loss or fetal anomalies in multiple pregnancies.
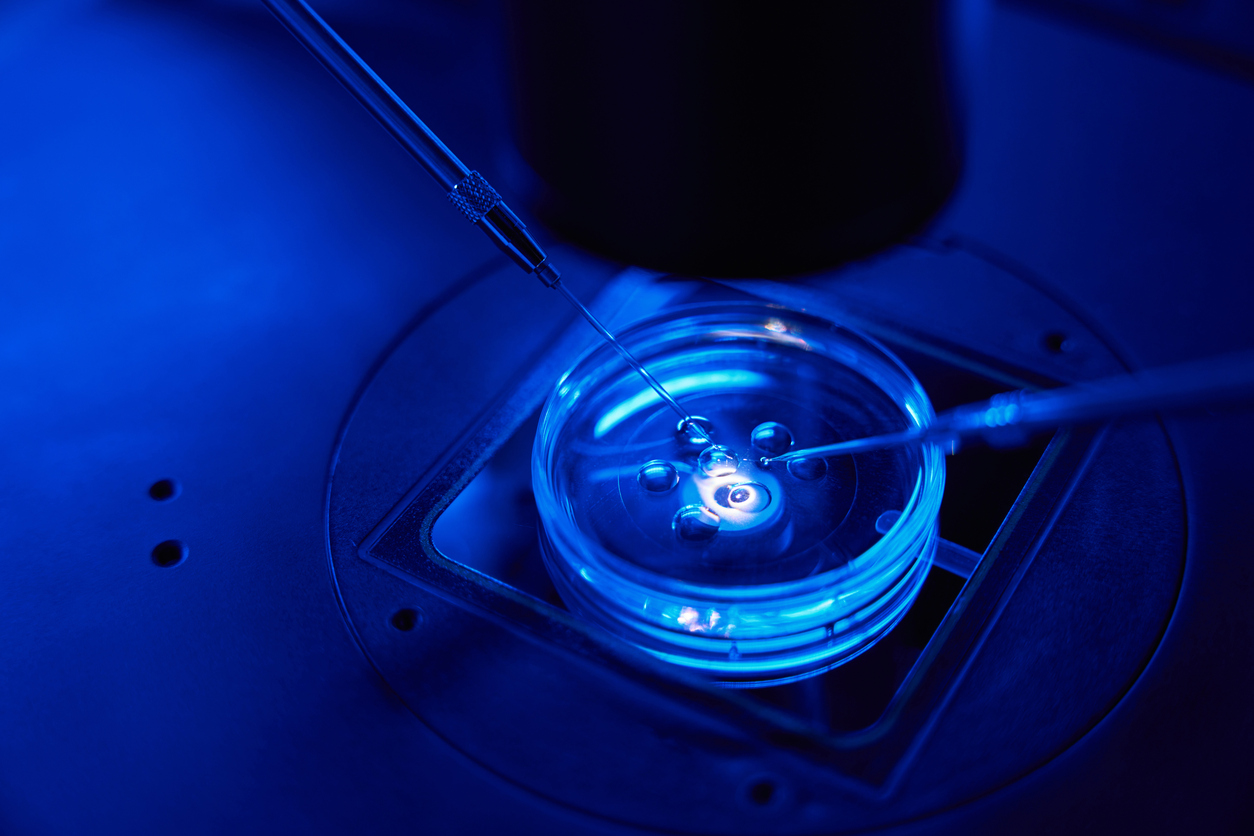
ICSI: INTRACYTOPLASMIC SPERM INJECTION
ICSI is an advanced technique that has revolutionized the treatment of male infertility, allowing for pregnancies that previously seemed impossible. It is also used in cases of low fertilization rate in conventional IVF. It involves the injection of a selected sperm directly into the cytoplasm of an egg. Each egg receives a single sperm injection. After this injection, an additional period of between 18 and 48 hours is needed for fertilization to occur. If fertilization occurs, the resulting embryos are cultured for several days before being transferred to the uterus.
Although ICSI has the potential to achieve a certain level of fertilization regardless of sperm quality, it is important to note that male subfertility may be related to numerical or structural chromosomal defects. Therefore, it is advisable for all couples who achieve pregnancies through ICSI to undergo prenatal testing, especially from a genetic perspective. In some cases of obstructive azoospermia, there is a higher incidence of cystic fibrosis in men, so screening or consultation with a geneticist is suggested before addressing more extreme male infertility treatments. Although subtle differences in sperm quality could influence embryonic viability and the rate of early abortions, these observations do not imply that the ICSI technique itself is the cause, as it simply facilitates fertilization by sperm that, under other circumstances, would not have successfully fertilized the egg.
ICSI IN INFERTILITY NOT RELATED TO MALE FACTORS
Today, ICSI is routinely applied in various clinical situations where conventional in vitro fertilization may be compromised. These situations include unexplained fertility, ovarian hyperstimulation with low-quality eggs, sperm samples with poor survival after freezing, insemination of frozen eggs, generation of embryos for preimplantation genetic studies, and cases with an extreme need to maximize normal fertilization, such as when the woman has few recovered eggs. In some cases, ICSI can rescue situations where conventional fertilization has failed. Although embryos fertilized late have a lower viability potential than those fertilized on time, they have led to successful births. ICSI has become a preferred and common insemination technique in IVF therapy.
ASSISTED HATCHING
It has been proposed that some viable embryos do not implant because they cannot escape from the gelatin layer surrounding the egg (zona pellucida) when they reach the blastocyst stage. Around the unfertilized egg is a layer of glycoprotein that protects and regulates normal fertilization. This layer, similar to gelatin, continues to protect the early embryo until, as a blastocyst, it fills with fluid and “hatches” out of the zona pellucida. Factors such as advanced maternal age can make the zona pellucida thicker, making it difficult for the embryo to be released. “Assisted hatching” involves creating a hole in the zona pellucida before transfer, allowing the embryo to escape more easily. Although this technique is experimental and has limitations, it has been used with infrared laser to create the hole.
EMBRYO BIOPSY
In summary, it is relevant to mention preimplantation genetic diagnosis (PGT), which allows for the genetic study of the embryo. This technique can detect severe genetic mutations or chromosomal errors and the sex of each embryo.